Chapter 7 of the eu gmps 7 10 through 7 15 contracts requires a quality agreement to define the responsibilities of the contract giver and contract acceptor contract manufacture and.
Medical device contract manufacturing agreement.
Involved in contract drug manufacturing can use quality agreements to delineate their.
Medical devices dietary supplements.
And good manufacturing.
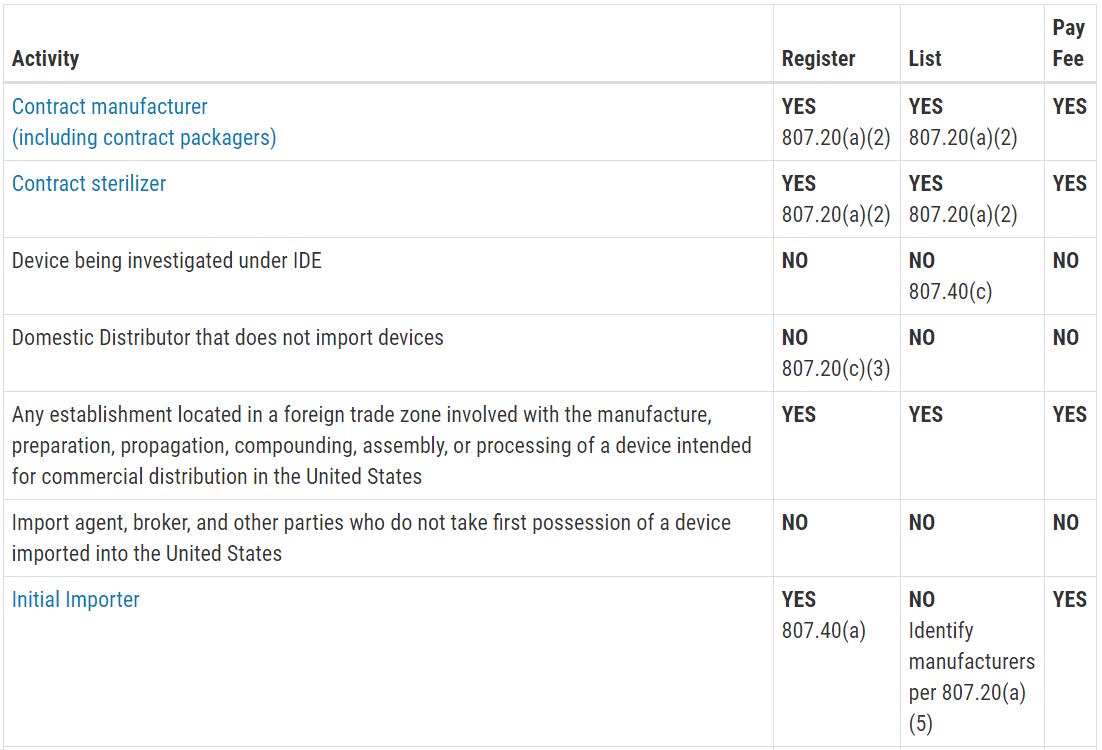
Quality agreements are required by medical device regulations 21 cfr 820 50 purchasing controls as well as various quality standards such as iso.
The contract manufacturing agreement should require that contract manufacturers assist in product and process documentation and plant inspections before and after regulatory approval.
Indemnification in the event the collaboration deteriorates or is challenged in any manner the medical device oem should ensure its interests are protected.
A big part of a medical device contract manufacturer is meeting their quality agreement s contractual obligations.
After the recent announcement and publication of article 47 of directive 2001 83 ec on the community code relating to medicinal products for human use and article 51 of directive 2001 82 ec for comments from industry contract manufacturing organizations cmo s from asia are facing renewed regulatory heat.
This agreement focuses on oem medical device customer clauses for manufacturing terms pricing payment terms purchase orders liability and forecasting initial and subsequent rescheduling ems provider delivery v oem acceptance manufacturing device changes product warranty customer equipment and components contract termination quality fda 510 k responsibility insurance and more.
This manufacturing agreement the agreement is entered into as of this 18th day of may 2005 by and between tissuelink medical inc a delaware corporation having its principal place of business at 1 washington center suite 400 dover nh 03820 the customer and phase ii medical manufacturing inc having its.
Medical device inspection contract program fact sheet version april 2019.
In the field of medical devices this is an especially important factor since to market said devices requires regulatory compliance.
Comprehensive written agreement between parties involved in the contract manufacturing of drugs that defines and establishes each party s manufacturing activities in terms of how each will comply with cgmp.
Parties that perform one or more manufacturing operations on behalf of an owner or owners.
Whereas seller is a contract manufacturer and is in the business of manufacturing and assembling medical device components and subassemblies for its customers and has considerable engineering development and manufacturing experience in the area of injection molded plastic and metal parts.